The Activation Energy Is Best Described by
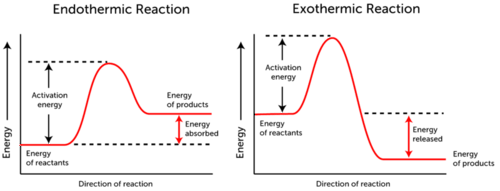
The energy required to end a chemical reaction the energy required to bind a substrate to an active site the energy required to form or break the bonds of reactant molecules the energy required to re-form bonds in product molecules. Activation energy is the minimum amount of energy needed to start a chemical reaction.

1 The Graph Below Represents The Potential Energy
For cellular reactions to occur fast enough over short time scales their activation energies are lowered by molecules called catalysts.

. The minimum amount of additional energy needed by a reacting molecule to get transformed into the product is termed activation energy. Activation energy in chemistry the minimum amount of energy that is required to activate atoms or molecules to a condition in which they can undergo a chemical transformation or physical transport. KJ and the reaction is D.
The activation energy is 10. A type of exergonic reaction b. The energy required for a reaction to proceed by breaking bonds.
This is the starting energy before we go on the reaction. Which of the following best describes activation energy. A numerical description of the amount of energy needed by colliding reactant molecules in order to form.
How do temperature and activation energy relate to. Energy difference between the reactants and the products. A type of endergonic reaction c.
The energy required to break the bonds of reactant molecules is. Rank the grades of coal by their relative desirabilities starting with mos desirable at the top. Question and answer.
The reaction is exothermic in the forward reaction. The energy required for a reaction to proceed by breaking bonds d. The diagram represents the electrolysis of hydrochloric acid HCl to form hydrogen gas H2 and chlorine gas Cl2.
This is the currently selected item. Use the diagram to answer the question. The source of the activation energy needed to push reactions forward is typically heat energy from the surroundings.
Activation energy transition state and reaction rate. Which is the best definition of activation energy. 2 marks Reaction Type DH Value A.
Activation Energy Barrier Recall that the activation energy represents the minimum amount of energy required to overcome the energy barrier. White phosphorus has a lower activation energy than red phosphorus. B the minimum kinetic energy that particles must possess for a chemical reaction to occur.
The energy added by a catalyst B. Activation energy is best described as ___ the energy required to initiate a chemical reaction. KJ and the reaction is endothermic.
The energy possessed by the products C. In simple terms it is the amount of energy that needs to be supplied in order for a chemical reaction to proceed. 3White phosphorus has a lower activation energy than red phosphorus.
So I think its C. Up to 24 cash back Which statement correctly describes the energy changes that occur in the forward reaction. Consider the following experimental results.
Because activation energy is the amount of energy required for a chemical reaction to take place. The temperature at which the reaction proceeds most quickly. Only a small fraction of the collisions between reactant molecules convert the reactants into the products of the reaction.
The difference in free energy between. C the energy of motion. The activation energy is 10.
The Activation Energy of Chemical Reactions. Energy difference between the reactants and the activated complex. The difference in free energy between the reactants and products of the reaction D.
The difference in free energy between the products and the transition state. Google Classroom Facebook Twitter. KJ and the reaction is C.
This can be understood by turning once again to the reaction between ClNO 2 and NO. Enzyme structure and catalysis. Energy of the activated complex.
Provides the activation energy for the reaction. Activation energy can be described as the a. The activation energy in the Arrhenius equation can best be described as asked Jun 26 2017 in Chemistry by maju88 a.
The catalysts needed to raise a reactions rate. Up to 24 cash back 1 forward activation energy kJ 20 2 reverse activation energy kJ 30 Which of the following describes the reaction type and enthalpy change for the forward reaction. Environmental impacts on enzyme function.
Enzymes and the active site. KJ and the reaction is. The energy needed for a reaction to occur D.
2energy stored in chemical bonds. D the energy required to separate ions in a crystalline solid. Which of the following best describes the activation energy of a chemical reaction.
1energy input needed to break bonds of reactants. 4How can the chemical potential energy in an endothermic reaction best be described1 point Reactants have higher chemical potential energy than products. Activation energy is the amount of energy required to reach the transition state.
The activation energy is 50. Which best describes the energy of activation. This energy must be supplied from the collision energy of the reactant molecules.
Enzyme structure and catalysis. Which statement best describes the reaction. The formation of the products releases bond energy and this energy is less than the energy.
The minimum amount of energy needed for a reaction to potentially occur 1 See answer Advertisement Advertisement frostydiamond5676 frostydiamond5676. The activation energy is 50. White phosphorus has a higher activation energy than red phosphorus.
4Products have higher chemical potential energy than reactants. Which statement best describes the reaction.

Activation Energy Article Khan Academy

No comments for "The Activation Energy Is Best Described by"
Post a Comment